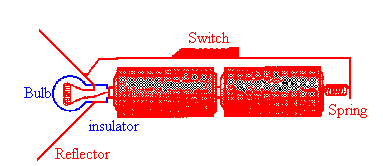
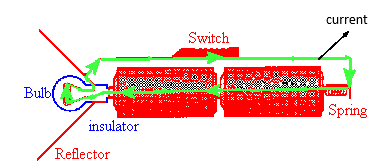
Note how the current flows through the bulb!
A basic flashlight has four components, a battery, a bulb, a switch and a metal strip, that connects the other components together. Electric current flows from the negative to the positive terminal of the battery through the battery, through the filament of the bulb, and through the switch and metal strip back to the negative terminal in one continuous circuit. (The electrons which carry the current actually flow in a continuous circuit in the opposite direction.)


Note how the current flows through the bulb!
A typical alkaline battery pumps electrons until the potential difference between its positive and negative terminals is 1.5 V. Electrochemical reactions provide the energy that an alkaline battery converts into electrical energy. Powdered zinc attached to the negative terminal forms a zinc-oxide complex with a manganese dioxide paste attached to the positive terminal. The reaction process resembles controlled combustion. In effect, the battery "burns" zinc to obtain the energy needed to pump electrons from the negative to the positive terminal.
Often two batteries are connected together in series, so that the positive terminal of one battery touches the negative terminal of the other. Each battery pumps charges until its positive terminal is 1.5 V above its negative terminal. Therefore the chain's positive terminal is 3.0 V above its negative terminal. The voltages of the two batteries add because each battery adds energy to the charges as it pumps them through its interior. The more batteries are in series, the greater is the potential difference between the chain's positive terminal and its negative terminal. If one of the batteries in a chain is reversed, this battery will extract energy from the current passing through it. If a chain has 3 batteries, 2 of them will add energy to the electrons while the one that is reversed extracts energy from the electrons. The potential difference across the chain then is only 1.5 V.
Rechargeable batteries can turn some of the energy they extract from a current flowing backwards through their interior back into chemical potential energy. Normal alkaline batteries are not rechargeable. They turn most of the energy they extract from the recharging current into thermal energy instead of chemical potential energy. Non-rechargeable batteries may overheat and explode during recharging.
The bulb's filament is a coil of fine tungsten wire, which has a large resistance R. The metal strips connecting the batteries and the bulb have negligible resistance. As a current I = V/R flows through the circuit, electrical energy is converted into thermal energy and light in the bulb. The power output is P = IV.
A typical current for a flashlight with two batteries in series is 1.0 A. The resistance of the bulb determines the current. If a 1.0 A current flows through a flashlight with two batteries in series, the flashlight consumes 3 W of power. The bulb has been designed to become white hot when it dissipates 3 J of energy per second. If the wrong bulb is put into a flashlight, it will dissipate too much or too little energy per second. If too much current flows, the bulb will burn out quickly.
A total of 600 C of charge passes through a flashlight in 0.500 h. What
is the average current?
Solution:
External link: PhET Simulation: Circuit Construction Kit: DC